USI-CRC Study Group
USI-CRC Study Group
Introduction Urological Society of India (USI) is the premier professional organization of Urologists in India. One of the chief objectives of USI is the advancement of urological care. Collaborative research is integral to this goal. Collaborative research enables more representative geographical inclusion of data, expedites recruitment of an adequate sample size and leverages the scientific acumen of the Indian Urological community. It is able to provide answers to clinical questions at an epidemiological level and is applicable on a larger scale to the masses. Urological society of India aims to lay the foundations for high-quality collaborative research under the umbrella of the USI through the establishment of a Collaborative Research Committee of the USI (CRC-USI).
Objectives Collaborative Research under the USI will be encouraged with intent of improving our understanding of urological conditions, diseases or practice as important to Indian Urology or Indian Urologists.
- This includes but is not limited to
- Diseases that have a high burden in India
- Diseases that are specifically common in India but less common globally
- Development of diagnostic modalities or algorithms for such conditions
- Issues on which Indian Urologists are recognized to have special expertise
- Research to address lacuna in Indian data
- Novel hypotheses
- Systematic reviews on issues of specific relevance to Indian Urology
CRC-USI approach
The Collaborative Research activities will be started with research that does not involve a clinical trial. These research activities are planned as confidence building exercises to give the program a strong academic start. No trial directed interventional studies will be undertaken in this first phase. Only a limited number of projects will be undertaken in Phase one. The initial number is envisaged as being 10 projects in the first year.
- The following collaborative research is envisaged in Phase I:
- Data collection studies
- Small studies, retrospective, cross-sectional or prospective observational data collection.
- Audits
An important goal of Phase I is to demonstrate our ability to conduct research as an organization with an adherence to rigorous and global scientific standards. Emphasis will be on stringent monitoring of projects. This is essential to establish a benchmark for the future. The CRC-USI recognizes the critical role of setting an initial standard. Toward this goal, monitoring of objective parameters for the research by Nodal Officers will not be at the discretion of either the researchers or the Nodal Officers themselves but will be subject to direct oversight of the CRC-USI.
CRC-USI Team and structure Collaborative Research Committee of USI (CRC-USI):
The Committee consists of a chairman and four senior members and 5 junior members appointed by the USI Council and has a term of 3 years. Members have been identified based on their academic credentials as well as their ability and willingness to be a part of this project.
CRS-USI Core team:
Members:
Dr. Nitin Kekre (Chairman)
Dr. Shrawan Kumar Singh
Dr. Sujatha Patwardhan
Dr. Sanjay Sinha
Dr. Amit Ghosh
Dr. Prashant Nayak
Dr. Rishi Nayyar
Dr. Karthikeyan
Dr. Bhavatej Enganti
Dr Ashwin Tamhankhar

Procedure for Submission of Research protocols
Principal Investigator
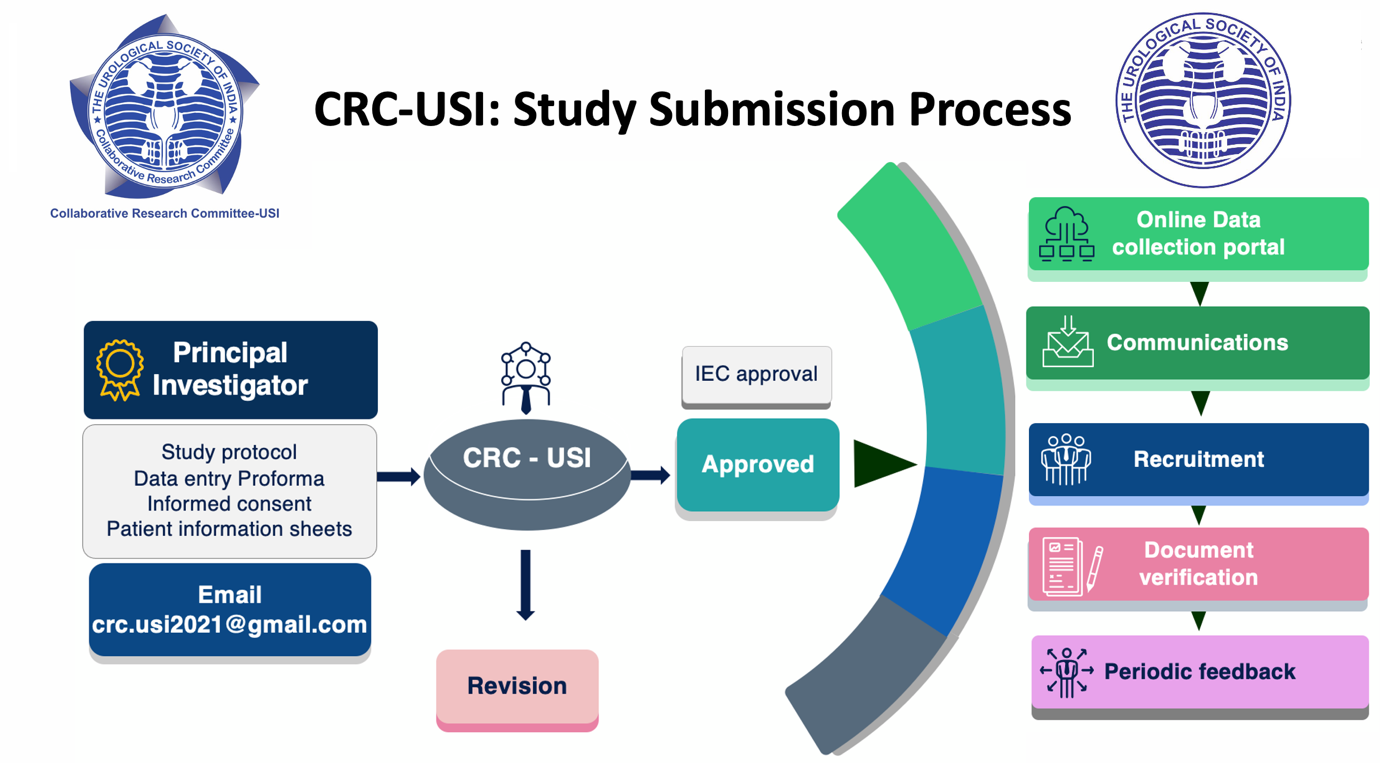
If a member (i.e., Principal investigator (PI)) decides to perform a multi-center collaborative study. The PI can approach or communicate the committee and submit the study protocol to the following e-mail: [email protected]
There is no specific time frame for submission of such proposals and all USI members interested in collaborative research are encouraged to send in their proposals in brief as and when they are ready with study protocol (Title, Introduction, Aims and Objectives, Proposed methodology, Data collection Performa). Informed consent and patient information sheets (including vernacular language) shall also be needed once project is approved.
The study will be presented to the committee for approval. If there are revisions, the committee will suggest accordingly. Once the study protocol is approved, the PI needs to get IEC approval. The study approval will be communicated to USI. USI IT team will coordinate with the PI and provide a central database platform for data entry and storage pertaining to the study. Meanwhile, PI will communicate and inform other members for enrollment into the study through USI. The study protocol will be published in the USI newsletter and communicated officially to all the members through e-mail. PI can personally communicate and take help from committee if necessary. Members interested in the study will contact the PI of the study for approval. PI periodically needs to inform the committee regarding the members or institutes enrolled and the timely progress of the study.
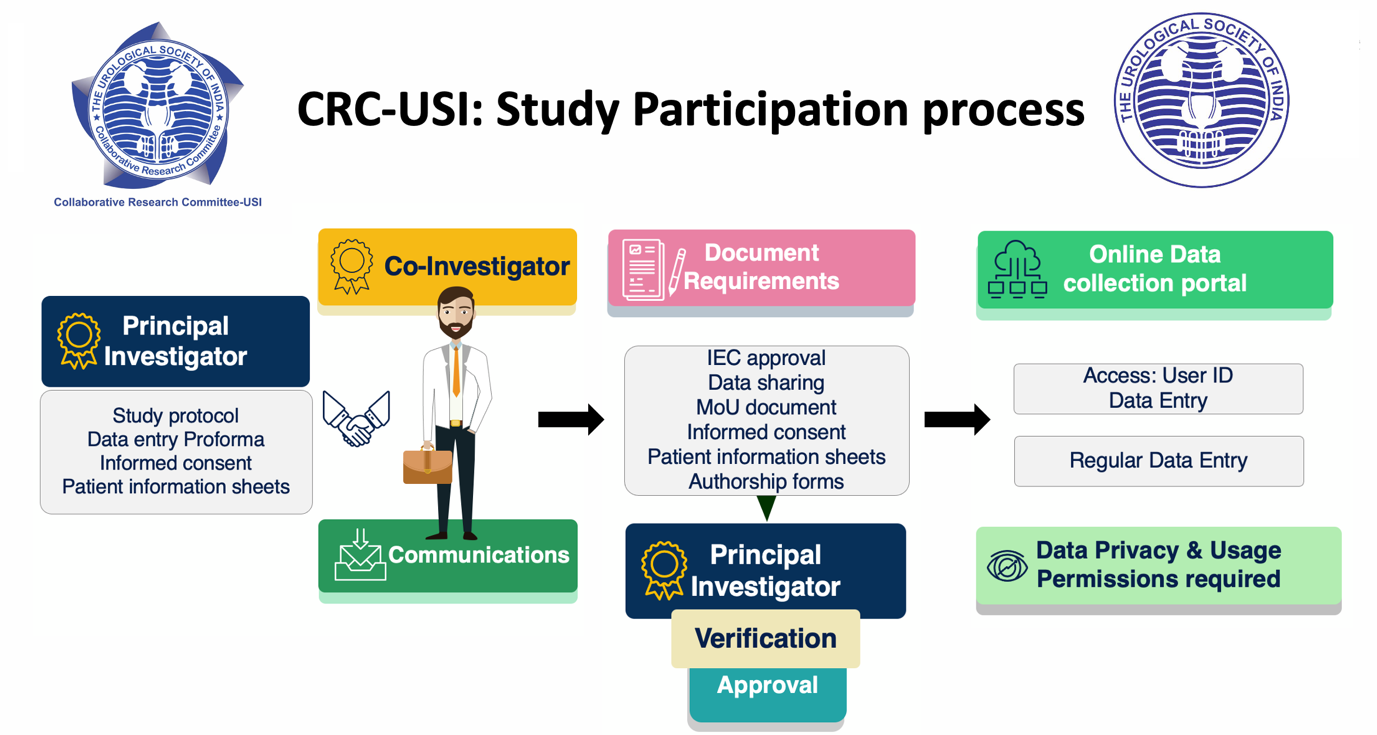
Co-Investigator
Members (i.e., Co-investigators) who are keen to enrol in the study need to communicate with the PI. PI shall provide all the necessary documents required for ethical approval at each individual participating center of the study. The co-investigators need to get IEC approval, sign a Letter of Understanding or authorship related documents and the terms and conditions pertaining to the study before enrolment. Once the verification is confirmed by the PI, a secure access for online data entry will be provided. Regular entry of data is required. The Co-investigator will have access to his or their institute data only.
Approved Projects
Study 1:
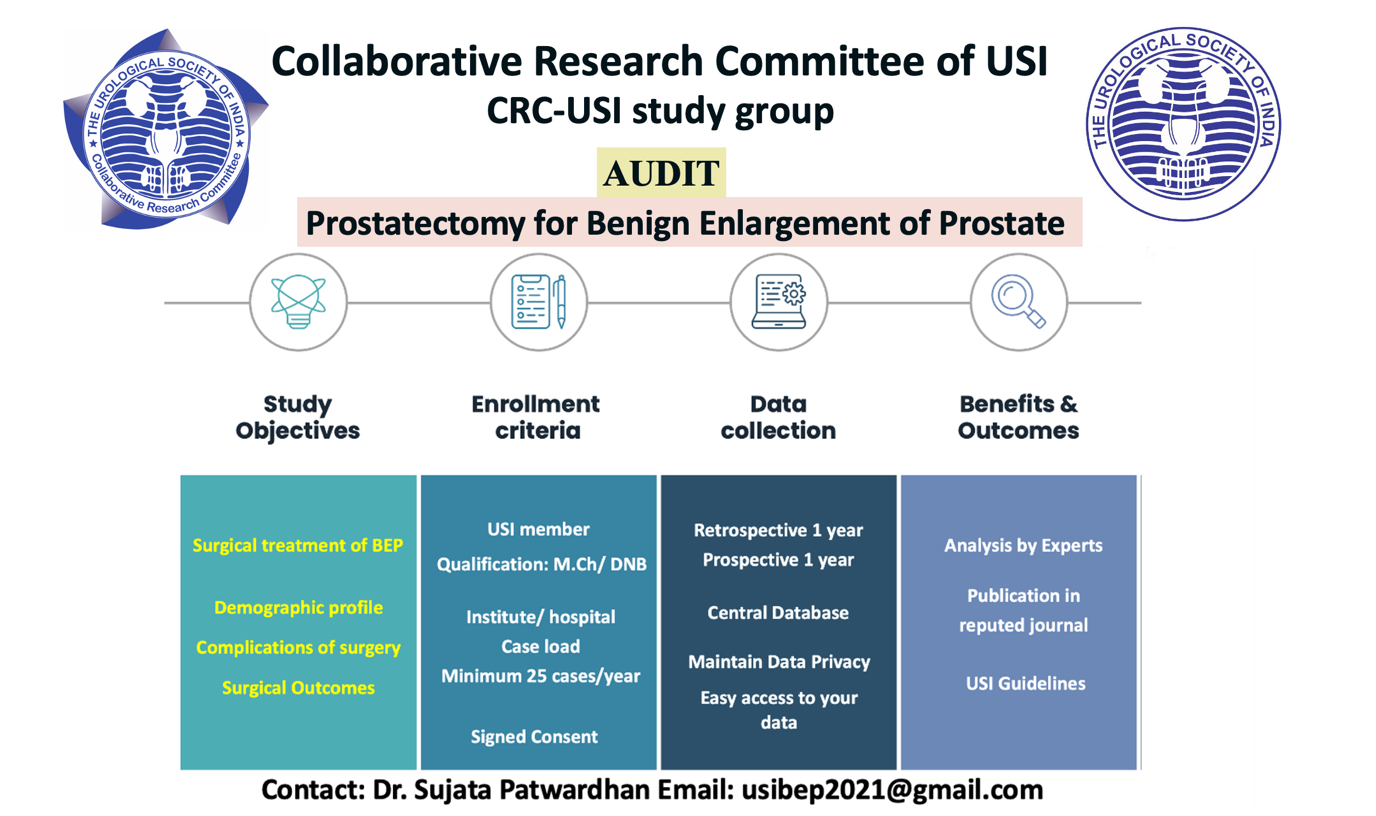
Project Title: Prostatectomy for Benign enlargement of prostate
Principal investigator: Dr Sujata Patwardhan, KEM Hospital, Mumbai.
Status: Ongoing
Contact Email: [email protected]
Click here for Project Details
Click here for Clinical Data Entry
Click here for Study Project Progress
Study 2:
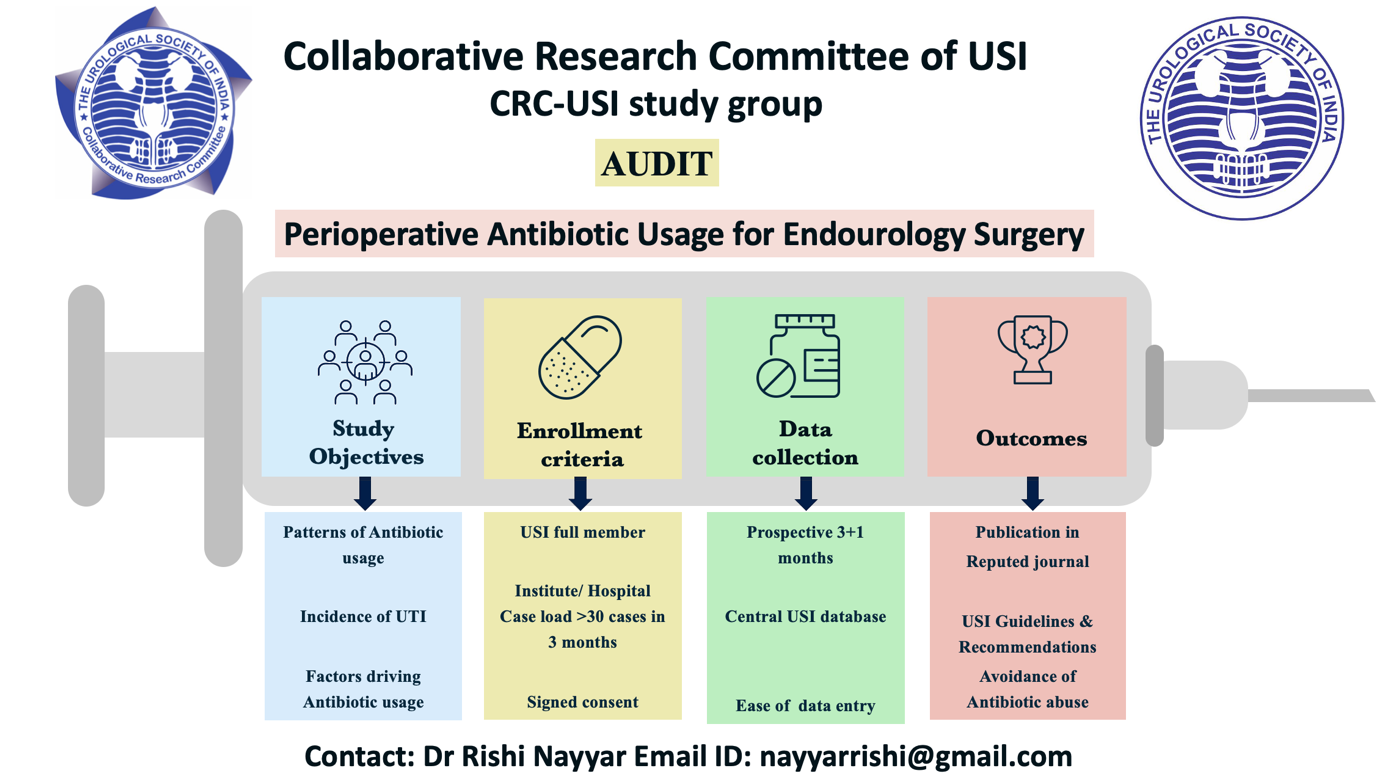
Project Title: Perioperative antibiotic usage for Endourology surgery
Principal investigator: Dr Rishi Nayyar, AIIMS, New Delhi.
Status: Ongoing
Contact Email: [email protected]
Click here for Project Details
Click here for Clinical Data Entry
Click here for Study Project Progress
Primary Contact for Protocol submissions:
Contact us: [email protected]